New Particulate Matter Testing Services
-
Upload
-
0
-
Embed
-
Share
-
Upload and view presentations on any device and embed the player to your website! --- > >Upload PPT
- Upload PPT
Presentation Transcript
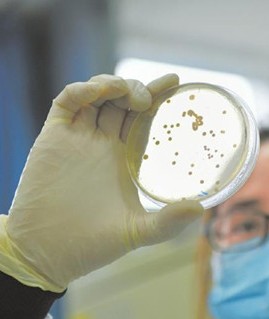
- 1.New Particulate Matter Testing Services STEMart, a US-based provider of comprehensive services for all phases of medical device development, is proud to announce the launch of its new Particulate Matter Testing Services with a wide range of particulate matter testing from <0.001 μ m to >3,000 μm. STEMart can provide accurate analysis and material characterization from nanometer scale particles to bulk powders with its various testing techniques. Particulate matter is defined as foreign, mobile, undissolved particles, excluding air bubbles, unintentionally present in injectable drugs, parenteral infusions, ophthalmic solutions and various medical devices. Particulate Matter testing is used to remove, count, and size particulate contaminants that are inherent to the product or that result from the manufacturing process. This testing is useful for identifying potential sources of particulate matter and avoiding potential risks to patients from the device. STEMart now offers Particulate Matter Testing Services with a range of particulate matter testing from <0.001 μm to >3,000 μm. The team of experts can provide accurate analysis and material characterization from nanometer scale particles to bulk powders using various testing techniques. STEMart's Particulate Matter Testing uses two methods, the Light Obscuration Particle Count test and Microscopic Particle Count test. The former test is adapted from United States Pharmacopeia Method <788>, which provides standards for injectable solutions, uses an appropriate instrument based on the principle of light masking that automatically determines the size of the particles and the number of particles based on size. The size ranges used for USP testing are ≥10 μm and ≥ 25 μ m. Liquid particle counters are capable of measuring and counting particles from 2 μm to 100 μm in diameter. The general procedure for Microscopic Particle Count test is to wet the inside of a filter holder containing a membrane filter with a few milliliters of particle-free water, transfer the total volume of the solution cell or individual units to a filter funnel and apply a vacuum, then place the filter in a Petri dish and allow the filter to air dry. After the filter has air dried, place the Petri dish on the stage of the microscope, scan the entire membrane filter under the reflected light of the illumination unit, and count the number of particles. This procedure is also under the guidance of USP Method <788>, which provides standards for injectable solutions, and the size ranges used for USP testing are ≥10 μm and ≥25 μm. To learn more about STEMart's Particulate Matter Testing Services, please visit the website at https://www.ste-mart.com/. About STEMart
- 2.STEMart is an industry-leading eCommerce platform incorporated with an extensive global footprint and a broad portfolio of more than 10,000 products. It aims to provide better lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage upward to the manufacturing process. STEMart is dedicated to enhancing research and biotech production with simpler and safer protocols in order to access better health worldwide.
Related