Disinfection Validation Services
-
Upload
-
0
-
Embed
-
Share
-
Upload and view presentations on any device and embed the player to your website! --- > >Upload PPT
- Upload PPT
Presentation Transcript
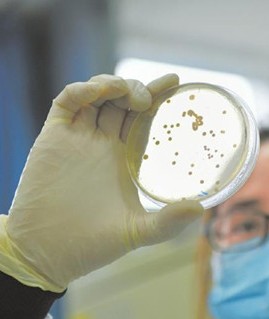
- 1.STEMart Launches Disinfection Validation Services for Reusable Medical Devices STEMart, a U.S.-based provider of comprehensive services for all phases of medical device development, is pleased to announce that it offers Disinfection Validation Services for reusable medical devices. These services are designed to ensure that products meet FDA's disinfection validation requirements and patient safety. Cleaning validation services must be performed on reusable instruments, trays, surgical kits and similar items that have been used in patient care. Manufacturers of reusable medical devices must provide reprocessing instructions describing cleaning, disinfection and sterilization and prove that these procedures are effective. Medical Device Disinfection Validation is used to verify the manufacturer's description of the disinfection steps to demonstrate the effectiveness of the microbicidal process. Disinfection validation studies should focus on "worst case" scenarios to show that end users can reliably disinfect devices when instructions are followed. STEMart now offers Disinfection Validation Services for reusable medical devices to ensure customer products meet FDA’s disinfection validation requirements and patient safety. The validation processes are in compliance with AAMI TIR12, ANSI/AAMI ST58, ISO 15883 series, ISO 17664, and FDA guidance documents. STEMart will not only help customers validate specific programs, but also advise them on the design and creation of protocols for their reusable devices. STEMart now offers Disinfection Validation Services for reusable medical devices to ensure that customer products meet FDA requirements for disinfection validation and patient safety. These validation processes comply with AAMI TIR12, ANSI/AAMI ST58, ISO 15883 series, ISO 17664 and FDA guidance documents. STEMart not only assists customers with the validation of specific programs, but also consults with them on the design and creation of protocols for their reusable devices. Disinfection refers to the use of physical or chemical methods to kill microorganisms, usually accomplished through chemical disinfectants or thermal disinfection. Validation of chemical disinfection is performed in a variety of ways to support high-level, intermediate-level and low-level disinfection, depending on the required disinfection leverage. The level of disinfection is determined by the category of the device (critical, semi-critical, or non-critical). These validations require that the device be inoculated with bacteria and then immersed in a liquid chemical disinfectant solution. Finally, any remaining bacteria are extracted from the device and grown on plates in a manner similar to a bioburden test. The other type of disinfection, thermal disinfection, utilizes hot water from 60°C to 95°C (140°F to 203°F). The level of thermal disinfection depends on the contact
- 2.time and temperature. If you are looking for professional disinfection validation services for your reusable medical devices, or would like to know more about STEMart's medical device development service, please visit https://www.ste-mart.com/. About STEMart STEMart is an industry-leading eCommerce platform incorporated with an extensive global footprint and a broad portfolio of more than 10,000 products. It aims to provide better lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage upward to the manufacturing process. STEMart is dedicated to enhancing research and biotech production with simpler and safer protocols in order to access better health worldwide.
Related