Flow Cytometry Technical Tips and Calibration Particles
-
Upload
-
0
-
Embed
-
Share
-
Upload and view presentations on any device and embed the player to your website! --- > >Upload PPT
- Upload PPT
Presentation Transcript
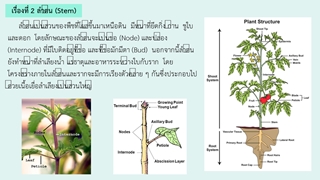
- 1.Flow Cytometry: Technical Tips and Calibration Particles Figure 1. Flow cytometry is routinely used in basic research, clinical practice, and clinical trials. CD Determine Cell Characteristics & Function Diagnosis of Health Disorders Such as Blood Cancers Protein Engineering Detection Detect Microorgan- isms Biomarker Detection Flow cytometry is a technique used to detect and measure the physical and chemical characteristics of a group of cells or particles (Figure 1). In this process, a group of cells or particles is suspended in a liquid and then injected into a flow cytometer. Ideally, one cell at a time flows through the laser beam, and the light scattered in the laser beam is unique to the cell and its components. Cells are usually labeled with fluorescence so that the light is absorbed and emitted into a wavelength band. Tens of thousands of cells can be quickly detected and the data collected is processed by a computer. Flow cytometry is an instrument that provides quantitative data. Similar to flow cytometry, cell sorters can physically separate and purify cells of interest based on their optical characteristics. Cell Counting Cell Sorting Tel: 1-631-633-6938 Email: info@cd-bioparticles.com
- 2.Tips for Fluorophore Selection Tel: 1-631-633-6938 Email: info@cd-bioparticles.com Technical Tips for Choosing Flow Cytometry Antibodies Try commonly used clone numbers. For a specific CD (clusters of differentiation) molecule, there are usually several monoclonal antibodies with different clone numbers. The more often a clone is used for flow cytometry, the greater the chance that the experiment will be successfully completed.
- 3.High SI (staining index) ensures good separation of positive and negative cell populations, especially in experiments that require high resolution. Although isotype antibodies or blocking agents are commonly used, suboptimal coupling of fluorescent dyes to antibodies significantly exacerbates background binding, especially for antibodies labeled by a labeling kit without purification. Low background binding makes it much easier to identify positive and negative cell populations. Due to their size and inability to cross the plasma membrane efficiently, tandem dyes are recommended for extracellular staining only. For direct labeling of antigens, it is recommended to use conjugated antibodies rather than paired primary and secondary antibodies. Common buffer additives can interfere with the coupling reaction and limit the reaction efficiency. Custom conjugated antibodies with BSA and azide-free packaging may be required. When using a conjugated antibody, the ratio of fluorochrome to protein (F: P, represents the degree of labeling) of the fluorescent dye and the protein of interest should be calculated. For direct labeling of antigens, it is recommended to use conjugated antibodies rather than paired primary and secondary antibodies. Common buffer additives can interfere with the coupling reaction and limit the reaction efficiency. Custom conjugated antibodies with BSA and azide-free packaging may be required. When using a conjugated antibody, the ratio of fluorochrome to protein (F: P, represents the degree of labeling) of the fluorescent dye and the protein of interest should be calculated. Indirect detection is more sensitive and important for effectively identifying low-abundance antigens and rare epitopes. If no signal is received after using an unconjugated primary antibody, check the species of the secondary antibody. For indirect detection, the cross-species reactivity of secondary antibodies is often a problem. Antibody labeling kits eliminate the need to use secondary antibodies, resulting in reduced number of incubation and washing steps while eliminating background caused by cross-species reactions. Tips for Reducing High Background Fluorescence It is best to use fresh cells or cells with a shorter fixing time to reduce the risk of autofluorescence leading to high background fluorescence. It is recommended to run matched unstained cells with the sample to assess autofluorescence. It is strongly recommended to use viability dyes such as PI, DAPI,7-AAD, Annexin V and pSIVA to account for non-specific binding. Tissue dissociation and digestion often lead to cell death and high background fluorescence, so it is important to distinguish between viable and dead cells during analysis (Figure 2). Tel: 1-631-633-6938 Email: info@cd-bioparticles.com
- 4.Figure 2. Annexin V/PI staining guidelines. Increase buffer capacity, the number of washes, and/or wash times, especially if high background is observed when using unconjugated primary antibodies. Alternatively, the antibody titer may be too high and further dilution of the antibody may be required. When facing high background staining, using Fc receptor blocking reagents can avoid unwanted bindings between Fc region of the antibody and the Fc-receptors. Increasing the concentration or exposure time of such reagents would help too. The use of detergents can cause high background staining. For intracellular targets, the use of alcohol permeabilization is a good alternative. Tips for No Signal or Weak Fluorescence Intensity If the signal is weak, the detection antibodies may be too diluted. Although primary antibodies have been validated for flow cytometry, the specific cells, tissue types, or experimental conditions may require titration of antibody concentration. If no signal is detected, the target may be not accessible. Check the predicted location of the protein and whether the fixation and permeabilization methods are correct for the target. To prevent the internalization of surface antigens, cells should be kept on ice during the assay. In some cases, you can optimize the staining effect by adjusting the incubation temperature or staining time. If there is no problem with protocols of target fixation and permeabilization, and the optimal antibody titer has been determined under specific experimental conditions, verify if any pretreatment of the cells (such as stimulating immune cells) is required to induce or enhance the target molecule expression. If the targets are secreted proteins, make sure inhibitors such as Brefeldin A or monensin are used. These compounds prevent the export of newly synthesized proteins by disrupting the ER-Golgi transport mechanism and eventually capture the proteins in their respective cellular compartments. These inhibitors are needed when evaluating cytokines. Tel: 1-631-633-6938 Email: info@cd-bioparticles.com
- 5.For adherent cells that use trypsin to separate cells from the surface, the cause of the weak signal may be related to the effect of trypsin on the expression of extracellular molecules. Sodium azide prevents the modulation and internalization of surface antigens. If cryopreserved cells are used, check if the target antigen is affected by the freezing and/or thawing process. Check the excitation and emission spectra of the fluorescent dyes used. Make sure all lasers are properly aligned, as misalignment can cause weak signals. The use of calibration beads can help calibrate instrument performance for each channel. Excessive light during the dyeing process results in photobleaching of fluorescent dyes and dissociation of tandem dyes. Make sure the sample is protected from light as much as possible. Calibration Particles for Flow Cytometry There are two important factors to keep in mind when using manufactured particles (i.e. calibration particles) instead of cells for flow cytometry. First, particles are not cells and do not necessarily scatter light like cells. Second, the fluorescence of particles may be similar to that of cells stained with a particular dye, but is almost never exactly the same. Aligning Particles When installing and building the instrument, manufacturers use particles with multiple sizes and/or fluorescence levels to ensure optical alignment. Moreover, personally track the performance of the instrument after installation and before preventive maintenance are necessary. When running the alignment particles, in order to obtain the same mean fluorescence intensity (MFI) reading, one needs to be aware of any changes in the applied voltage. If the reading deviation appears on all detectors of a given laser, it may indicate a loss of laser power. In addition, pay attention to the changes in the coefficient of variation (CV) of the particle population. An increase in CV means a reduction in sensitivity, and there may be a misalignment of the laser or problems with detection optics. Creative Diagnostics applies alignment particles that are used to check whether the flow path of the flow cytometry is aligned and the inside of the instrument is clean or clogged. DiagPoly™ Ultral Multiple Fluorescent Polystyrene Particles have enhanced UV and Far Red Fluorescence intensity than DiagPoly™ Multiple Fluorescent Polystyrene Particles, and the latter is suitable for alignment of FITC, PE, PE-TR, and PE-Cy5 channels. Counting Particles Counting particles are beads of various sizes with or without fluorescence. The key is that these particles are provided at a defined concentration. This allows the setup of a stop gate to get a certain number of events and use of those events to calculate the concentration of cells in the original sample. These counting particles come in fluorescent and non-fluorescent forms, but fluorescent beads are often the best. Fluorescence makes them very easy to gate in comparison to gating only scattered signals. Tel: 1-631-633-6938 Email: info@cd-bioparticles.com
- 6.Creative Diagnostics offers fluorescent particles of diameters from 0.05-1.9 µm with increased forward light scatter sensitivity. They are designed to characterize microparticles (0.5-0.9 µm), aquatic bacterial (0.2-0.6 µm), and platelets (0.9-3 µm), which provides a submicron size standardization tool for flow cytometers. We also have size standard particles with diameters range from 3.0-17.9 µm. They are a group of uniform and nonfluorescent polystyrene microspheres, which can provide reliable size control, and the cell size can be predicted by the forward light scattering (FSC) measurements. References: Crowley, L. C., Marfell, B. J., Scott, A. P., & Waterhouse, N. J. (2016). Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harbor Protocols, 2016(11), pdb-prot087288. Wang, L., & Hoffman, R. A. (2017). Standardization, calibration, and control in flow cytometry. Current protocols in cytometry, 79(1), 1-3. 5 Tel: 1-631-633-6938 Fax: 1-631-938-8221 Email: info@cd-bioparticles.com Address: 45-1 Ramsey Road, Shirley, NY 11967, USA For more information, view our website: www.cd-bioparticles.com
Related